Countries colored in brown represent about 3.8 billion people, more than half the world's population. All have a permissive or flexible policy on human embryonic stem cell research and all except the U.S. have banned by law human reproductive cloning. Population: M = million.
Map Explanation
* Turkey is among several countries in which no
specific regulations and guidelines have so far been defined by legal or governmental institutions for human embryonic stem cell research. Dr. Necati Findikli of Istanbul Memorial Hospital reported the first known derivation of human embryonic stem cells from donated blastocyst-stage embryos in Turkey in 2005. Reproductive Medicine Online 10 (5), 617-627, 2005.
Images and Video
![]() "permissive" = various embryonic stem cell derivation techniques including somatic cell nuclear transfer (SCNT), also called research or therapeutic cloning. SCNT is the transfer of a cell nucleus from a somatic or body cell into an egg from which the nucleus has been removed. [Options 4 & 5 in Walters, LeRoy, in References, below] Countries in this category include Australia, Belgium, China, India, Israel, Japan, Singapore, South Korea, Sweden, the United Kingdom and others. [Walters, LeRoy, National Academy of Sciences, 2004; University of Minnesota, 2007. See References, below] These countries represent a global population of more than 2.7 billion people.
"permissive" = various embryonic stem cell derivation techniques including somatic cell nuclear transfer (SCNT), also called research or therapeutic cloning. SCNT is the transfer of a cell nucleus from a somatic or body cell into an egg from which the nucleus has been removed. [Options 4 & 5 in Walters, LeRoy, in References, below] Countries in this category include Australia, Belgium, China, India, Israel, Japan, Singapore, South Korea, Sweden, the United Kingdom and others. [Walters, LeRoy, National Academy of Sciences, 2004; University of Minnesota, 2007. See References, below] These countries represent a global population of more than 2.7 billion people.![]() "flexible" = derivations from fertility clinic donations only, excluding SCNT, and often under certain restrictions. [Option 3 in Walters, LeRoy, in References, below: "Research is permitted only on remaining embryos no longer needed for reproduction."] Countries in this category include Brazil, Canada, France, Iran, South Africa, Spain, The Netherlands, Taiwan, and others. [Walters, LeRoy, National Academy of Sciences, 2004; University of Minnesota, 2007. See References, below] These countries represent a global population of more than 1 billion people.
"flexible" = derivations from fertility clinic donations only, excluding SCNT, and often under certain restrictions. [Option 3 in Walters, LeRoy, in References, below: "Research is permitted only on remaining embryos no longer needed for reproduction."] Countries in this category include Brazil, Canada, France, Iran, South Africa, Spain, The Netherlands, Taiwan, and others. [Walters, LeRoy, National Academy of Sciences, 2004; University of Minnesota, 2007. See References, below] These countries represent a global population of more than 1 billion people. ![]() Restrictive policy or no established policy. For restrictive policy see Options 1 & 2 in Walters, LeRoy in References, below. Restrictive policies range from outright prohibition of human embryo research to permitting research on imported embryonic stem cell lines only to permitting research on a limited number of previously established stem cell lines. Countries with a restrictive policy include Austria, Germany, Ireland, Italy, Norway, and Poland.
Restrictive policy or no established policy. For restrictive policy see Options 1 & 2 in Walters, LeRoy in References, below. Restrictive policies range from outright prohibition of human embryo research to permitting research on imported embryonic stem cell lines only to permitting research on a limited number of previously established stem cell lines. Countries with a restrictive policy include Austria, Germany, Ireland, Italy, Norway, and Poland.![]()
Click above for discussion of The Stem Cell Dilemma
on Hawaii Public Radio's public affairs program Town Square
![]()
Stem Cell Animation: RIKEN Center for Developmental Biology, Kobe, Japan.
![]()
Bingaman, The Honorable Jeff. Video of Speech on the floor of the U.S. Senate, April 11, 2007
![]()
Green, Ronald. Dartmouth News: The Ethics of Stem Cells
References
Countries with a permissive or flexible policy
"Stem Cells and the New 'Age of Discovery'" [PDF] AUTM Central Regional Meeting, Minneapolis, July 23, 2006General
Bioscience Network Inc. The World Stem Cell Map
The Hinxton Group: World Stem Cell Policies The Hinxton Group: Statement on Policies and Practices Governing Data and Materials Sharing and Intellectual Property in Stem Cell Science [PDF]
HumGen International. StemGen World Map
![]() International Legislation on Human Embryonic Stem Cell Research
International Legislation on Human Embryonic Stem Cell Research ![]() The ISSCR Guidelines for the Conduct of Human Embryonic Stem Cell Research, Feb. 1, 2007.
The ISSCR Guidelines for the Conduct of Human Embryonic Stem Cell Research, Feb. 1, 2007.
California Proposition 71 [PDF].
Map by William Hoffman. Selected talks:
"Stem Cells, Regenerative Medicine, and Clusters of Innovation in the Asia-Pacific Region" [PDF] Stem Cells Asia 2010, Seoul, Oct. 28, 2010
"Stem Cell Research: Evolving Policy for a New Science" [PDF] University of Minnesota Stem Cell Institute, Nov. 17, 2010
A leading resource for information about stem cell policy on ![]()
![]()
★Awarded a star by Kirkus Reviews
for "remarkable merit"
World Stem Cell Map cited by:
Beaver, Nathan and Matthew Mulkeen. Under the Microscope: The International Legal & Business Issues Surrounding the Stem Cell Initiative. Foley & Lardner LLP, Washington D.C. BioJapan 2005. September 8, 2005. PDF [2.3 MB]
Brito, Arturo. The Childrens Health Fund. "Stem Cell Research: The Ethics of Non-Action." September 1, 2007 PDF
Knowles, Lori. The Business of Regulating Stem Cell Research, American Enterprise Institute, March 9, 2005. Stem Cell Research Symposium, New England School of Law. November 19, 2004. Website
Bingaman, The Honorable Jeff. Speech on the floor of the U.S. Senate , April 11, 2007![]()
Bingaman, The Honorable Jeff. Video of Speech on the floor of the U.S. Senate, April 11, 2007 [short video]
Caplan, Arthur. Medical College of Virginia, Oct. 11, 2004 and various stem cell academic presentations and public lectures. Website
Department of Health, Catalonia, Spain. "Considerations concerning nuclear transfer," December 2005. [PDF]
Dinnetz, Mattias Karlsson. "Stem Cell Research, Science Policy and the
Emergence of an Academic Centre," Lund University, Sweden, 2006.
Dodd David A. "Stem Cell Science & Technology:
Commercialization Opportunities & Challenges," MIT Enterprise Forum of Atlanta,
October 12, 2006. [podcast]
Eisenstadter, Ingrid. "Blacklists and Blastocysts," Barron's, July 10, 2006.
Epstein, David. "Free For All, Inside Higher Ed, July 25, 2006.
Global Watch: Stem cell mission to China, Singapore and South Korea, Department of Trade & Industry, United Kingdom, September 2004. PDF
Green, Ronald M. "Embryo and Fetal Research" In: The Cambridge Textbook of Bioethics, Cambridge University Press, 2008.
Greenwood, Heather L. and Abdahlla S. Daar. "Regenerative Medicine" In: The Cambridge Textbook of Bioethics, Cambridge University Press, 2008.
Gross, Michael. "Framework bolsters stem cell progress." Current Biology, 14 (15): R592-R593, August 10, 2004.
House of Commons Library, UK Parliament, Research Paper 08/42, "Human Fertilisation and Embryology Bill," May 2, 2008
Hug, Christina. EuroStemCell Workshop - working paper, Lund University, Sweden, March 2006 [PDF]
Kadereit, Suzanne. Stem Cell Research Symposium, New England School of Law. November 19, 2004. Website (ISSCR)
Keane, Steve, The Case Against Blanket First Amendment Protection of Scientific Research: Articulating a More Limited Scope of Protection, Stanford Law Review: 59 (2) 505, 2006 [PDF]
![]()
Kirk, Mark, U.S. Congressman from the 10th Congressional District of Illinois. "Stem Cell Politics on Capitol Hill," BIO 2006, April 2006. PowerPoint
Latham, Stephen R. "Between public opinion and public policy: human embryonic stem-cell research and path-dependency." J Law Med Ethics 37(4): 800-6, 2009
Leist, Marcel et al. "The Biological and Ethical Basis of the
Use of Human Embryonic Stem Cells for
In Vitro Test Systems or Cell Therapy," Altex 25 (3) 2008, pp. 163-190. [PDF]
Levinson, Rachel. "How Policy is Made: Lessons from Current Issues," Biodesign Institute, Arizona State University, November 15, 2005. PowerPoint
McCabe, Linda L. and Edward R.B. McCabe.DNA: Promise and Peril, University of California Press, 2008
The Milken Institute. "Stem Cell Innovation: The Next-Frontier Economy?" California: State of the State Conference 2005: Renewing California's Global Leadership, October 31, 2005. [PDF - 4MB]
UNESCO - International Bioethics Committee Report of the Working Group of IBC on Human Cloning and International Governance
Ott, Marie-Odile. "Human Embryo and Embryonic Stem Cell Research in France: State of the Art and Analysis ," Center for American Progress, June 15, 2007 [PDF]
The Parliament of Victoria [Australia]: Therapeutic Cloning: The Infertility Treatment Amendment Bill 2007. Current Issues Brief No. 1, April 2007 [PDF]
Peters, Ted. "The Stem Cell Debate in America and Around the Globe," Collegium for Advanced Studies, University of Helsinki, 20 September 2007 [Doc]
Polina, Felipe. "Human Stem Cells - European National Innovation Systems and Patents," Lund University, Sweden, May 29, 2006
Salter, Brian. Evolution of the Life Science Industries: Policy and Regulation. Edinburgh, UK, February 23, 2005. Website
Taylor, Stacy. Patenting the Products of Stem Cell
Research: A Global Perspective. Foley & Lardner LLP, Washington D.C. BayBIO Stem Cell Program. September 19, 2005. PDF [1.1 MB]
Trounson, Alan. Molecular Medicine Symposium: Stem Cell Biology and Human Disease. Salk Institute. March 18, 2005. Website.
Walters, LeRoy. Public Policies on Human Embryonic Stem Cell Research: An Intercultural Perspective. National Academy of Sciences Workshop, October 12, 2004. Website
World Stem Cell Map published by:
Anatolia College Model United Nations 2008. Bioethics Committee Study Guides, 2008 [PDF]
Burrill's BIOTECH 2007 Life Sciences: A Global Transformation
Asahi Shimbun [Tokyo, Japan], Feb. 1, 2008 [PDF]
![]()
Biofutur: "Recherche sur les cellules souches," Marie-Odile Ott, January 2007 [PDF]
Burrill's BIOTECH 2008 Life Sciences: A 20/20 Vision to 2020
CV Network (International Academy of Cardiovascular Sciences), Fall 2004
![]()
"Global Culture"
Financial Times, "An industry to grow," June 25, 2009
![]()
Financial Times, "Bush's veto of embryo stem cell law marks turning point with Congress," July 20, 2006
Financial Times, "Stem cell researchers hope for $3 billion boost," Oct. 28, 2004
Hoffman, John, Stem Cells: Part 6: Medical Tourism: seeking cures around the world, Philadelphia Examiner, April 25, 2009.
Issues: Stem Cells by Peggy J. Parks, For: Compact Research: Current Issues, published by ReferencePoint Press, Fall 2008
Japan Science and Technology Agency - Center for Research and Development Strategy. G-TeC Report on Stem Cell Research, 2007 [PDF - in Japanese]
The Journal of Life Sciences, September 2007.
Mauron, A and ME Jaconi , "Stem cell science: Current ethical and policy issues," Nature - Clinical Pharmacology and Therapeutics. Advance online publication, July 18, 2007. [PDF]
Schmickle, Sharon, "Stem cell stalemate: Minnesota authors say U.S. falling behind other nations," MinnPost.com. March 25, 2008
![]()
Sword and Shield: Dual Uses of Pathogen Research, Jan. 5, 2011. What do stem cells have to do with bioterrorism?
![]()
The Monitor Group: Joseph Fuller and Brock Reeve: "National Competitiveness in Stem Cell Science," February 2007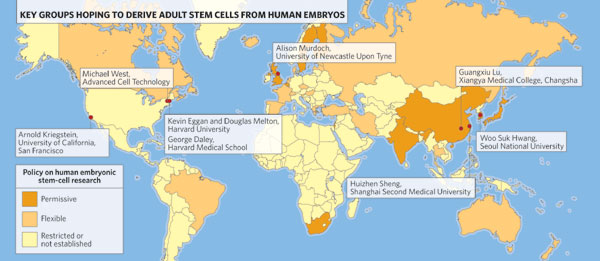
Nature, Dec. 22, 2005
Nature Biotechnology, July 2005 [Global Competitiveness / Stem Cell Research Map]
NeuroInsights: The Neurotechnology Industry 2005
New Jersey Star-Ledger, March 20, 2005
Public Library of Science: PLoS Biology, July 2005
Public Library of Science: PLoS Medicine, May 2006
![]()
Red Herring, June 20, 2005
Red Herring, November 20, 2006
![]()
San Diego Union-Tribune, Dec. 17, 2006
Science Actualités Cité des Sciences, Paris, March 18, 2005
Stem Cell Blogs:
California Stem Cell Report
Maps created with GMT software
World Stem Cell Map linked to by:
Wikipedia - Stem cell research policy
Science News, April 2, 2005
The Scientist, March 28, 2005.
UK Trade & Investment: "Global commercialisation of UK stem cell research" [PDF], Nicola Perrin, University of Cambridge, August 2005.
Stem Cell Network Blog
Knoepfler Lab Stem Cell Blog, UC Davis School of Medicine
Updated 1/7/13
National Institutes of Health - Stem Cell Information
American Association for the Advancement of Science - AAAS
Nature
the Niche: the stem cell blog, Nature
Nature Reports: Stem Cells, Nature
Scientific American editors' blog
International Society for Stem Cell Research - ISSCR
Federation of American Societies for Experimental Biology - FASEB
Harvard University Stem Cell Institute
Stem Cell Policy Aaron Levine, School of Public Policy, Georgia Institute of Technology
Coalition for the Advancement of Medical Research -- CAMR
The Globalism Institute - Royal Melbourne Institute of Technology, Australia
Com Ciência Brazil
International Academy of Cardiovascular Sciences [PDF] Canada
StemCellsChina.com China
EurActiv.com European Union
Science & Décision, Université d'Évry & Centre National de la Recherche Scientifique, France
Bioethik Discurs Berlin, Germany
Robert Koch Institut Germany
RegenerationNet.com STERN BioRegion, Germany
Tokugikon - Japanese Patent Office Society [PDF, in Japanese] Japan
National Health Foundation - Bioethics Thailand
UK Stem Cell Foundation United Kingdom
Research!America Stem cell research resources
Genetics Policy Institute
Northwest Association for Biomedical Research NWABR Stem Cell Teacher Workshop and Educator: Selected Online Resources for Stem Cells
Health Politics with Dr. Mike Magee
Science Friday National Public Radio
StemCellResources.org Bioscience Network in association with:
the Biology Teachers Association of NJ and the National Association of Biology Teachers
Results for America campaign
Center for American Progress
Grassroots Connection Online Neurological Advocacy
CareCure Community W. M. Keck Center for Collaborative Neuroscience at Rutgers University
Kirsch Foundation Medical Research
California Stem Cell Report
Great North Alliance Twin Cities Technology Resources
Massachusetts General Hospital
Indiana Center for Bioethics
Michigan eLibrary
Missouri Roundtable Ethical implications of biotechnological research
Canadian Prescription Drugstore
High School Bioethics Project University of Pennsylvania Center for Bioethics
Cosmic Log by Alan Boyle MSNBC, Jan. 4, 2006
The Future of Biotechnology for Medical Applications in 2005, Governmental Issues ScenarioThinking.org
Legal Restrictions for Biotech increasing in certain countries, decreasing in others ScenarioThinking.org
William Hoffman - hoffm003@umn.edu
Acknowledgments: Individuals who have provided foundational ideas, constructive criticism, encouragement or other input for the global bioscience maps include: Joseph Amato (Marshall, MN), Ivan Berkowitz (Winnipeg), William Brody (Baltimore), G. Steven Burrill (San Francisco), Arthur Caplan (Philadelphia), Rob Carlson (Seattle), Gareth Cook (Boston), Clive Cookson (London), David Cyranoski (Tokyo), David Durenberger (Minneapolis), Petr Dvorak (Czech Republic), Juan Enriquez (Rockville MD), Francis Fukuyama (Washington DC), Leo Furcht (Minneapolis), John Gearhart (Baltimore), William Gleason (Minneapolis), Ron Green (Dartmouth), Ginger Gruters (Washington, DC), Jon Hakim (Beijing), Michael Hoffman (Bloomington, MN), Suzanne Holland (Seattle), Abdul Latif Ibrahim (Malaysia), Marisa Jaconi (Geneva), William Johnson (Boston), Louis Johnston (Collegeville MN), Suzanne Kadereit (Singapore), Naoko Kimura (Bangkok), Lori Knowles (Edmonton), Zack Lynch (San Francisco), Stephen Minger (London), Martin Murphy (Durham NC), Thomas Murray (New York), William Neaves (Kansas City MO), Marie-Odile Ott (Paris), Robert Paarlberg (Wellesley, MA), Nicola Perrin (Cambridge UK), Douglas Petty (Minneapolis/St. Paul), Michael Porter (Boston), Walter Powell (Stanford), Clyde Prestowitz (Washington DC), John Rennie (New York), Kate Rubin (Minneapolis/St. Paul), G. Edward Schuh (Minneapolis/St. Paul), Lee Silver (Princeton), Peter Singer (Toronto), Doug Sipp (Kobe, Japan), Carl Sundberg (Stockholm), William Testa (Chicago), Alan Trounson (Melbourne), LeRoy Walters (Washington DC), Steven Weber (Berkeley), Sarah Youngerman (Minneapolis) and Laurie Zoloth (Chicago).
The Library of Congress
![]()
is preserving parts
of MBBNet through its
Web Archiving Project.